Aldehydes are not prone to reactions. Aldehydes. Properties. Receipt and use
Aldehydes are organic compounds in which the carbonyl group (C-O) is bonded to hydrogen and the radical R (residues of aliphatic, aromatic and heterocyclic compounds):
The polarity of the carbonyl group ensures the polarity of the molecule as a whole, so aldehydes have higher boiling points than nonpolar compounds of comparable molecular weight.
Since the hydrogen atoms in aldehydes are bonded only to the carbon atom (close relative electronegativity), no intermolecular hydrogen bonds are formed. Therefore, the boiling points of aldehydes are lower than those of the corresponding alcohols or carboxylic acids. As an example, we can compare the boiling points of methanol (T^ 65 °C), formic acid (Gbp 101 °C) and formaldehyde (7^, -21 °C).
Lower aldehydes are soluble in water, probably due to the formation of hydrogen bonds between the solute and solvent molecules. Higher aldehydes are highly soluble in most common organic solvents (alcohols, ethers). Lower aldehydes have a pungent odor, aldehydes with C3-C6 have a very unpleasant odor, while higher aldehydes have floral odors and are used in perfumery.
Chemically, aldehydes are very reactive compounds. The most typical reactions for aldehydes are nucleophilic addition reactions, which is due to the presence in the molecule of an electrophilic center - the carbonyl carbon atom of the C=0 group.
Many of these reactions, for example, the formation of oximes, semicarbazones and other compounds, are used in the qualitative and quantitative analysis of drugs from the aldehyde group because the addition products of aldehydes are characterized by a melting point specific for each aldehyde. Thus, aldehydes, when shaken with a saturated solution of sodium hydrogen sulfite, easily enter into an addition reaction:
The addition products are salts that have a certain melting point and are highly soluble in water, but insoluble in organic solvents.
When heated with dilute acids, hydrosulfite derivatives hydrolyze to their parent compounds.
The ability of aldehydes to form hydrosulfite derivatives is used both to determine the authenticity of a drug with an aldehyde group in the molecule, and to purify aldehydes and isolate them from mixtures with other substances that do not react with sodium hydrosulfite.
 |
|||||||||
Aldehydes also readily add ammonia and other nitrogen-containing nucleophiles. The addition products are usually unstable and easily undergo dehydration and polymerization. The cyclic compounds formed as a result of polymerization, when heated with dilute acids, easily decompose, again releasing the aldehyde:
| r-ch-nh2 | g z | -NH R-CC |
| -зн2о " | ||
| He |
Aldehydes are easily oxidized. Silver(I) oxide and other oxidizing agents with a low oxidation potential are capable of oxidizing aldehydes. For example, aldehydes are characterized by the formation of a silver mirror, which occurs with an ammonia solution of AgN03:
AgN03 + 3NH3 - OH + NH4N03
Tollens reagent
In this case, a mirror coating of metallic silver is formed on the walls of the test tube:
2OH + RCOH 2Agi + RCOOH + 4NH3T + H20
Likewise, aldehydes can reduce copper(II) to copper(I). To carry out the reaction, Fehling's reagent (an alkaline solution of copper(II) tartrate complex) is added to the aldehyde solution and heated. First, a yellow precipitate of copper(1) hydroxide, CuOH, is formed, and then a red precipitate of copper(1) oxide, Cu20:
2KNa + RCOH + 3NaOH + 2KOH -
2CuOHi + RCOONa + 4KNaC4H406 + 2H20 2CuOH - Cu20 + H20
The redox reaction also includes the reaction of aldehydes with Nessler's reagent in an alkaline medium; in this case, a dark precipitate of reduced mercury precipitates:
K2 + RCOH + ZKON - RCOOK + 4KI + Hgl + 2Н20
It should be borne in mind that the reaction with Nessler's reagent is more sensitive, so it is used to detect aldehyde impurities in drugs. The authenticity of drugs containing an aldehyde group is confirmed by less sensitive reactions: a silver mirror or with Fehling's reagent. Some other compounds, such as polyphenols, are also oxidized by Ag(I) and Cu(P) compounds, i.e. the reaction is not specific.
Formaldehyde and acetaldehyde are prone to polymerization. Formaldehyde polymerizes to form cyclic trimers, tetramers or linear polymers. The polymerization reaction occurs as a result of the nucleophilic attack of the oxygen of one molecule of the carbonyl carbon atom of another:
Thus, from a 40% aqueous solution of formaldehyde (formalin) a linear polymer is formed - paraform (u = 8 - 12), trimer and tetramer.
Aldehydes are characterized by narcotic and disinfectant properties. Compared to alcohols, the aldehyde group increases the toxicity of the substance. The introduction of a halogen into the aldehyde molecule increases its narcotic properties. For example, the narcotic properties of chloral are more pronounced than those of acetaldehyde:
![]() s!3s-ss
s!3s-ss
Receipt. Aldehydes can be obtained by oxidation of primary alcohols with chromic acid (Na2Cr04, H2S04) at boiling or with potassium permanganate in an alkaline medium:
Dehydrogenation of primary alcohols is carried out over a copper catalyst (Cu, Cr2O3) at 300-400 °C.
The industrial production of methanal is based on the vapor-phase oxidation of methanol with an iron-molybdenum catalyst:
2CH3OH + 02 500 ~600 2CH2=0 + H20
Formaldehyde solution (formalin)
Receipt. Formalin is an aqueous solution of formaldehyde (40%) stabilized with methanol (6-10%). The European Pharmacopoeia contains the FS “Formaldehyde solution (35%)” (see Table 9.1). In laboratory conditions, formaldehyde can be obtained by dehydrogenation of methanol over copper or depolymerization of paraform.
Determination of authenticity. Pharmacopoeial method - silver mirror reaction.
Since formaldehyde easily enters into condensation reactions, for example, with hydroxyl-containing aromatic compounds to form colored compounds, the State Fund also recommends using a reaction with salicylic acid for its identification, which results in a red color:
|
||||||||||
|
||||||||||
|
||||||||||
The reaction with chromotropic acid proceeds similarly with the formation of blue-violet and red-violet products (EP).
To determine the identity of pharmaceuticaldehyde, reactions with nitrogen-containing nucleophiles, such as primary amines, can be used:
H-Ctf° + H2N-R - n-s^^K + H20
The resulting N-substituted imines (Schiff bases) are slightly soluble, some of them are colored, others give colored compounds with heavy metal ions. EF suggests a reaction with phenylhydrazine. In the presence of potassium ferricyanide in an acidic environment, intensely red reaction products are formed.
Purity tests. Control of formic acid impurities is carried out by determining acidity. According to the Global Fund, the concentration of formic acid in the preparation should not exceed 0.2%; The content of formic acid is determined by the neutralization method (NF). According to the EF, methanol is determined by gas chromatography (9-15% vol.). Sulfated ash - no more than 0.1% in a 1.0 g sample.
I2 + 2NaOH - Nal + NaOI + H20
Hypoiodite oxidizes formaldehyde to formic acid. When the solution is acidified with excess sulfuric acid, unreacted hypoiodite is converted into iodine, which is titrated with sodium thiosulfate:
НСО + NaOI + NaOH - HCOONa + Nal + H20 NaOI + Nal + H2S04 -*■ I2 + Na2S04 + H20 I2 + 2Na2S203 - Na2S406 + 2NaI
It is possible to use other titrating agents in the determination of formaldehyde: hydrogen peroxide in an alkaline solution, cerium (IV) sulfate, sodium sulfite.
The drug can be considered as a prodrug, since the physiological effect is not exerted by hexamethylenetetramine itself, but by formaldehyde, which is released when the drug decomposes in an acidic environment. This is precisely why it is included in this section (see Table 9.1).
Receipt. Hexamine (tetraazaadamantane) is obtained by condensation of methanal and ammonia from aqueous solutions. The reaction intermediate is hexahydro-1,3,5-triazine:
| ll Hexahydro-Hurotropine 1,3,5-trnazine |
Determination of authenticity. When heating a mixture of the drug with diluted sulfuric acid, an ammonium salt is formed, from which ammonia is released when an excess of alkali is added:
(CH2)6N4 + 2H2S04 + 6H20 - 6HSON + 2(NH4)2S04 (NH4)2S04 + 2NaOH - 2NH3t + Na2S04 + 2H20
Hexamethylenetetramine can also be detected by the red coloration of the solution when salicylic acid is added after preheating with sulfuric acid (see identification of formaldehyde).
Purity tests. The presence of impurities of organic compounds, paraform, and ammonium salts is not allowed in the preparation. The State Fund specifies the permissible limits for the content of impurities of chlorides, sulfates, and heavy metals.
Quantitation. For the quantitative determination of hexamethylenetetramine, the GF suggests using the neutralization method. To do this, a sample of the drug is heated with an excess of 0.1 M sulfuric acid solution. Excess acid is titrated with an alkali solution with a concentration of 0.1 mol/l (methyl red indicator).
The iodometric method of quantitative determination is based on the ability of hexamethylenetetramine to give tetraiodides with iodine.
The first group of properties are addition reactions. In the carbonyl group, there is a double bond between carbon and oxygen, which, as you remember, consists of a sigma bond and a pi bond. In addition reactions, the pi bond is broken and two sigma bonds are formed, one with carbon and the other with oxygen. A partial positive charge is concentrated on carbon, and a partial negative charge on oxygen. Therefore, a negatively charged reagent particle, an anion, is attached to carbon, and a positively charged part of the molecule is attached to oxygen.
First property hydrogenation, addition of hydrogen.
The reaction occurs when heated. The already known hydrogenation catalyst nickel is used. Primary alcohols are obtained from aldehydes, and secondary alcohols from ketones.

In secondary alcohols, the hydroxo group is bonded to a secondary carbon atom.
Second property hydration, addition of water. This reaction is only possible for formaldehyde and acetaldehyde. Ketones do not react at all with water.
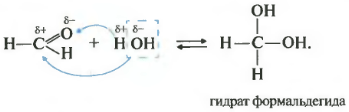
All addition reactions proceed in such a way that plus goes to minus, and minus to plus.
As you remember from the video about alcohols, the presence of two hydroxo groups on one atom is an almost impossible situation; such substances are extremely unstable. So these two specific cases - hydrate of formaldehyde and acetaldehyde - are possible, although they exist only in solution.
It is not necessary to know the reactions themselves. Most likely, the question on the exam may sound like a statement of fact, for example, substances react with water and are listed. Among them, the list may include methanal or ethanal.
Third property addition of hydrocyanic acid.

Again, plus goes to minus, and minus to plus. The resulting substances are called hydroxynitriles. Again, the reaction itself is not common, but it is a property you should be aware of.
Fourth property addition of alcohols.
Here again, you don’t need to know the reaction equation by heart, you just need to understand that such an interaction is possible.

As usual in reactions of addition to a carbonyl group, plus to minus, and minus to plus.
Fifth property reaction with sodium hydrosulfite.

And again, the reaction is quite complex, it is unlikely that you will be able to learn it, but this is one of the qualitative reactions to aldehydes, because the resulting sodium salt precipitates. That is, in fact, you should know that aldehydes react with sodium hydrosulfite, this will be enough.
This concludes with the first group of reactions. The second group is polymerization and polycondensation reactions.
2. Polymerization and polycondensation of aldehydes
You are familiar with polymerization: polyethylene, butadiene and isoprene rubbers, polyvinyl chloride - these are products of combining many molecules (monomers) into one large, single polymer chain. That is, one product is obtained. During polycondensation, the same thing happens, but in addition to the polymer, low molecular weight products are also obtained, for example, water. That is, two products are obtained.
So, sixth property polymerization. Ketones do not enter into these reactions; only the polymerization of formaldehyde is of industrial importance.
The pi bond breaks and two sigma bonds are formed with neighboring monomers. The result is polyformaldehyde, also called paraform. Most likely, the exam question may sound like this: substances enter into polymerization reactions. And there is a list of substances that may include formaldehyde.
The seventh property is polycondensation. Once again: during polycondensation, in addition to the polymer, a low molecular weight compound is also obtained, for example, water. Formaldehyde reacts with phenol in this way. For clarity, we first write the equation with two phenol molecules.

As a result, such a dimer is formed and a water molecule is split off. Now let's write the reaction equation in general form.

The polycondensation product is phenol-formaldehyde resin. It has a wide range of applications, from adhesives and varnishes to plastics and chipboard components.
Now the third group of properties - oxidation reactions.
3. Oxidation of aldehydes and ketones
Eighth the reaction in the general list is a qualitative reaction to the aldehyde group - oxidation with an ammonia solution of silver oxide. The “silver mirror” reaction. I will say right away that ketones do not enter into this reaction, only aldehydes.

The aldehyde group is oxidized to a carboxyl, acidic group, but in the presence of ammonia, which is a base, a neutralization reaction immediately occurs and the salt ammonium acetate is obtained. The silver precipitates, coating the inside of the test tube and creating a mirror-like surface. This reaction occurs on the Unified State Exam all the time.
By the way, the same reaction is qualitative for other substances that have an aldehyde group, for example, formic acid and its salts, as well as glucose.
Ninth the reaction is also qualitative to the aldehyde group oxidation with freshly precipitated copper hydroxide two. Here I will also note that ketones do not enter into this reaction.

Visually, the formation of a yellow precipitate will first be observed, which then turns red. In some textbooks there is information that first copper hydroxide one is formed, which has a yellow color, which then breaks down into red copper oxide one and water. So this is not true - according to the latest data, during the process of precipitation, the size of the copper oxide particles changes, which ultimately reach sizes that are colored red. The aldehyde is oxidized to the corresponding carboxylic acid. The reaction occurs very often on the Unified State Examination.
Tenth reaction: oxidation of aldehydes with an acidified solution of potassium permanganate when heated.
The solution becomes discolored. The aldehyde group is oxidized to a carboxyl group, that is, the aldehyde is oxidized to the corresponding acid. For ketones, this reaction has no practical meaning, since the molecule is destroyed and the result is a mixture of products.
It is important to note that formic aldehyde, formaldehyde, is oxidized to carbon dioxide, because its corresponding formic acid is itself not resistant to strong oxidizing agents.
As a result, carbon goes from oxidation state 0 to oxidation state +4. Let me remind you that methanol, as a rule, under such conditions is oxidized to a maximum of CO 2, skipping the stage of both aldehyde and acid. This feature must be remembered.
Eleventh reaction combustion, complete oxidation. Both aldehydes and ketones burn to carbon dioxide and water.
Let us write the reaction equation in general form.
According to the law of conservation of mass, there should be as many atoms on the left as there are atoms on the right. Because in chemical reactions, atoms do not disappear, but the order of bonds between them simply changes. So there will be as many molecules of carbon dioxide as there are carbon atoms in the molecule of a carbonyl compound, since the molecule contains one carbon atom. That is, n CO 2 molecules. There will be two times fewer water molecules than hydrogen atoms, that is, 2n/2, which means just n.
There are the same number of oxygen atoms on the left and right. On the right there are 2n of carbon dioxide, because each molecule has two oxygen atoms, plus n water, for a total of 3n. On the left there are the same number of oxygen atoms 3n, but one of the atoms is in the aldehyde molecule, which means it must be subtracted from the total to get the number of atoms per molecular oxygen. It turns out that 3n-1 atoms contain molecular oxygen, which means there are 2 times fewer molecules, because one molecule contains 2 atoms. That is (3n-1)/2 oxygen molecules.
Thus, we have compiled an equation for the combustion of carbonyl compounds in general form.
And finally twelfth property related to substitution reactions halogenation at the alpha carbon atom. Let us turn once again to the structure of the aldehyde molecule. Oxygen pulls electron density onto itself, creating a partial positive charge on carbon. The methyl group tries to compensate for this positive charge by displacing electrons from the hydrogen to it through a chain of sigma bonds. The carbon-hydrogen bond becomes more polar and the hydrogen breaks off more easily when attacked by a reagent. This effect is observed only for the alpha carbon atom, that is, the atom next to the aldehyde group, regardless of the length of the hydrocarbon radical.
In this way, it is possible to obtain, for example, 2-chloroacetaldehyde. Further substitution of hydrogen atoms to trichloroethanal is possible.
(for the simplest aldehyde R=H)
Classification of aldehydes
According to the structure of the hydrocarbon radical:
Limit; For example:

Unlimited; For example:

Aromatic; For example:

Alicyclic; For example:

General formula of saturated aldehydes

Homologous series, isomerism, nomenclature

Aldehydes are isomeric to another class of compounds, ketones.

For example:

Aldehydes and ketones contain a carbonyl group ˃C=O and are therefore called carbonyl compounds.
Electronic structure of aldehyde molecules
The carbon atom of the aldehyde group is in a state of sp 2 hybridization, therefore all σ bonds in this group are located in the same plane. Clouds of p electrons forming a π bond are perpendicular to this plane and are easily displaced towards the more electronegative oxygen atom. Therefore, the C=O double bond (unlike the C=C double bond in alkenes) is highly polarized.
Physical properties

Chemical properties
Aldehydes are reactive compounds that undergo numerous reactions. Most characteristic of aldehydes:
a) addition reactions at the carbonyl group; HX type reagents are added as follows:

b) oxidation reactions of the C-H bond of the aldehyde group, resulting in the formation of carboxylic acids:
I. Addition reactions
1. Hydrogenation (primary alcohols are formed

2. Addition of alcohols (hemiacetals and acetals are formed)

In excess alcohol in the presence of HCl, hemiacetals are converted to acetals:

II. Oxidation reactions
1. The “silver mirror” reaction

Simplified:

This reaction is a qualitative reaction to the aldehyde group (a mirror coating of metallic silver is formed on the walls of the reaction vessel).
2. Reaction with copper(II) hydroxide

This reaction is also a qualitative reaction to the aldehyde group y (a red precipitate of Cu 2 O precipitates).
Formaldehyde is oxidized by various O-containing oxidizers, first to formic acid and then to H 2 CO 3 (CO 2 + H 2 O):

III. Di-, tri- and polymerization reactions
1. Aldol condensation

2. Trimerization of acetaldehyde

3. Polymerization of formaldehyde
During long-term storage of formaldehyde (40% aqueous solution of formaldehyde), polymerization occurs in it with the formation of a white paraform precipitate:

IV. Polycondensation reaction of formaldehyde with phenol

Aldehydes are organic substances that belong to carbonyl compounds containing the functional group -SON, which is called a carbonyl group.
Depending on the nature of the hydrocarbon skeleton, aldehyde molecules are saturated, unsaturated and aromatic. Their molecules may also include halogen atoms or additional functional groups. The general formula of saturated aldehydes is C n H 2 n O. In accordance with the IUPAC nomenclature, their names end with the suffix -al.
The oxidation of aldehydes is important in industry because they are quite easily converted into carboxylic acids. In this case, copper hydroxide, silver oxide, or even air oxygen can serve as oxidizing agents.
Structure of the carbonyl group
The electronic structure of the double bond in the C=O group is characterized by the formation of one σ-bond and another π-bond. The C atom is in a state of sp 2 hybridization, the molecule has a flat structure with bond angles between bonds of about 120 0. The difference between the double bond in this functional group is that it is located between a carbon atom and a very electronegative oxygen atom. As a result, electrons are attracted to the O atom, which means that this bond is very highly polarized.
The content of such a polarized double bond in the aldehyde group can be called the main reason for the high reactivity of aldehydes. For aldehydes, the most typical reactions are the addition of atoms or their groups to the C=O bond. And the easiest reactions to occur are nucleophilic addition. Also typical for aldehydes are reactions involving H atoms from the functional group of aldehydes. Due to the electron-withdrawing effect of the C=O group, the polarity of the bond increases. This in turn is the reason for the relatively easy oxidation of aldehydes.
Individual representatives of aldehydes
Formaldehyde (formaldehyde or methanal) CH 2 O is a gaseous substance with a very pungent odor, which is usually obtained by passing a mixture of methanol vapor with air through a hot copper or silver mesh. Its 40% aqueous solution is called formalin. Formaldehyde readily reacts, many of which form the basis for the industrial synthesis of a number of important substances. It is also used to produce pentaerythritol, many medicinal substances, various dyes, for tanning leather, and as a disinfectant and deodorant. Formaldehyde is quite toxic; its maximum permissible concentration in the air is 0.001 mg/l.
Acetaldehyde (acetaldehyde, ethanal) CH 3 COH is a colorless liquid with a suffocating odor, which when diluted with water acquires a fruity aroma. Acetaldehyde has all the basic properties of aldehydes. The oxidation of acetaldehyde produces huge volumes of acetic acid and acetic anhydride, a variety of pharmaceuticals.
Acrolein (propenal) CH 2 =CH-SON, the simplest unsaturated aldehyde is a colorless, highly volatile liquid. Its vapors strongly irritate the mucous membranes of the eyes and upper respiratory tract. It is very toxic, the maximum permissible concentration for its content in the air is 0.7 mg/m 3. Propenal is an intermediate product in the synthesis of some polymers and is necessary in the production of certain medications.

Benzaldehyde (benzoaldehyde) C 6 H 5 COH is a colorless liquid with an aroma that turns yellow during storage. It is quickly oxidized by air to benzoic acid. Contained in essential oils of plants (neroli, patchouli), and in the form of a glucoside - in the kernels of bitter almonds, cherries, apricots and peach. As an aromatic substance, it is used in perfumery, as a component of food essences, and as a raw material for the synthesis of other aromatic substances (cinnamaldehyde, jasminaldehyde).
Silver mirror reaction
The oxidation of aldehydes by silver oxide is the most indicative qualitative reaction to the corresponding form of the functional group. This reaction got its name due to the thin silver coating on the walls of the test tube that forms during this reaction.
Its essence lies in the interaction of the aldehyde R-СОН with an ammonia solution of silver(I) oxide, which is a soluble OH complex compound and is called Tollens’ reagent. The reaction is carried out at temperatures close to the boiling point of water (80-100 °C). In this case, the aldehydes are oxidized to their corresponding carboxylic acids, and the oxidizing agent is reduced to metallic silver, which precipitates.
Preparation of reagents
To qualitatively determine the -SON group in aldehydes, a silver complex compound is first prepared. To do this, pour a little solution of ammonia (ammonium hydroxide) in water into a test tube, followed by a small amount of silver nitrate. In this case, the resulting silver oxide precipitate immediately disappears:
2AgNO 3 + 2NH 3 + H 2 O -> Ag 2 O↓ + 2NH 4 NO 3
Ag 2 O + 4NΗ 3 + Η 2 O -> 2ОΗ
More reliable results are obtained by Tollens' reagent prepared with the addition of alkali. To do this, 1 g of AgNO 3 is dissolved in 10 g of distilled water and an equal volume of concentrated sodium hydroxide is added. As a result, a precipitate of Ag 2 O is formed, which disappears when a concentrated solution of ammonium hydroxide is added. Only freshly prepared reagent should be used for the reaction.

Reaction mechanism
The reaction of a silver mirror corresponds to the equation:
2OΗ + HCOΗ -> 2Ag↓ + ΗCOONΗ 4 + 3NΗ 3 + H 2 O
It is worth noting that for aldehydes this interaction has not been sufficiently studied. The mechanism of this reaction is unknown, but a radical or ionic version of oxidation is assumed. With diammine silver hydroxide, the addition most likely occurs to form a silver diol salt, from which silver is then cleaved to form a carboxylic acid.
For a successful experiment, the cleanliness of the utensils used is extremely important. This is due to the fact that the colloidal silver particles formed during the experiment must adhere to the surface of the glass, creating a mirror surface. In the presence of the slightest contaminants, it will fall out in the form of a gray flaky sediment.
Alkaline solutions should be used to clean the container. So, for these purposes, you can take a NaOH solution, which needs to be washed off with a large volume of distilled water. There should be no grease or mechanical particles on the surface of the glass.
Oxidation with copper hydroxide
The oxidation reaction of aldehydes with copper(II) hydroxide is also quite spectacular and effective in determining the type of functional group. It proceeds at a temperature corresponding to the boiling of the reaction mixture. In this case, aldehydes reduce divalent copper in the composition of Fehling's reagent (freshly prepared ammonia solution of Cu(OH) 2) to monovalent copper. They themselves are oxidized due to the introduction of an oxygen atom into the C-H bond (the oxidation state of C changes from +1 to +3).
The progress of the reaction can be visually monitored by the change in color of the solution mixture. The bluish precipitate of copper hydroxide gradually turns yellow, corresponding to cuprous hydroxide and the further appearance of a bright red precipitate of Cu 2 O.
This process corresponds to the reaction equation:
R-SON + Cu 2+ + NaOH + H 2 O -> R-COONa + Cu 2 O + 4H +

Action by Jones reagent
It is worth noting that this reagent works best on aldehydes. In this case, oxidation does not require heating and is carried out at a temperature of 0-20 ° C for a fairly short period of time, and the yield of products is more than 80%. The main disadvantage of the Jones reagent is the lack of high selectivity for other functional groups, and besides, the acidic environment sometimes leads to isomerization or destruction.
Jones' reagent is a solution of chromium(VI) oxide in dilute acetone. It can also be obtained from sodium dichromate. When aldehydes are oxidized, carboxylic acids are formed under the influence of this reagent.
Industrial Oxidation with Oxygen
Oxidation of acetaldehyde in industry is carried out by exposure to oxygen in the presence of catalysts - cobalt or manganese ions. First, peracetic acid is formed:
CH 3 -SON + O 2 -> CH 3 -COOON
It, in turn, interacts with the second molecule of acetaldehyde and, through a peroxide compound, produces two molecules of acetic acid:
CH 3 -COOON + CH 3 -SON -> 2CH 3 -COOH
Oxidation is carried out at a temperature of 60-70 °C and a pressure of 2·10 5 Pa.
Interaction with iodine solution
To oxidize aldehyde groups, a solution of iodine in the presence of alkali is sometimes used. This reagent is of particular importance in the process of carbohydrate oxidation, since it acts very selectively. So, under its influence, D-glucose is converted into D-gluconic acid.

Iodine in the presence of alkalis forms hypoiodide (a very strong oxidizing agent): I 2 + 2NaOΗ -> NaIO + NaI + H 2 O.
Under the influence of hypoiodide, formaldehyde is converted into methane acid: ΗСОΗ + NaIO + NaOΗ -> ΗCOONa + NaI + H 2 O.
Oxidation of aldehydes with iodine is used in analytical chemistry to determine their quantitative content in solutions.
Oxidation with selenium dioxide
Unlike previous reagents, under the influence of selenium dioxide, aldehydes are converted into dicarbonyl compounds, and glyoxal is formed from formaldehyde. If methylene or methyl groups are located next to the carbonyl, they can be converted into carbonyl groups. Dioxane, ethanol or xylene are usually used as a solvent for SeO2.
According to one of the methods, the reaction is carried out in a three-neck flask connected to a stirrer, thermometer and reflux condenser. To the starting substance, taken in an amount of 0.25 mol, a solution of 0.25 mol of selenium dioxide in 180 ml of dioxane, as well as 12 ml of H 2 O, is added dropwise. The temperature should not exceed 20 ° C (if necessary, cool the flask). After this, with constant stirring, the solution is boiled for 6 hours. Next, the hot solution is filtered to separate selenium and the precipitate is washed with dioxane. After vacuum distillation of the solvent, the residue is fractionated. The main fraction is selected in a wide temperature range (20-30 °C) and rectified again.

Autoxidation of aldehydes
Under the influence of atmospheric oxygen at room temperature, the oxidation of aldehydes occurs very slowly. The main products of these reactions are the corresponding carboxylic acids. The autoxidation mechanism is similar to the industrial oxidation of ethanal to acetic acid. One of the intermediate products is a peracid, which reacts with another aldehyde molecule.
Due to the fact that this type of reaction is accelerated by light, peroxides, and traces of heavy metals, it can be concluded that it has a radical mechanism. Formaldehyde in aqueous solutions is much worse than its counterparts in being oxidized by air, due to the fact that it exists in them in the form of hydrated methylene glycol.

Oxidation of aldehydes with potassium permanganate
This reaction occurs most successfully in Visually, its progress can be assessed by the loss of intensity and complete discoloration of the pink color of the potassium permanganate solution. The reaction takes place at room temperature and normal pressure, so it does not require special conditions. It is enough to pour 2 ml of formaldehyde and 1 ml of acidified with sulfuric acid into the test tube. The test tube with the solution must be carefully shaken to mix the reagents:
5CH 3 -SON + 2KMnO 4 + 3H 2 SO 4 = 5CH 3 -COOH + 2MnSO 4 + K 2 SO 4 + 3H 2 O
If the same reaction is carried out at elevated temperatures, then methanal is easily oxidized to carbon dioxide:
5CH 3 -SON + 4KMnO 4 + 6H 2 SO 4 = 5CO 2 + 4MnSO 4 + 2K 2 SO 4 + 11H 2 O
Organic drugs
We study drugs divided into groups according to chemical classification. The advantage of this classification is the ability to identify and study general patterns in the development of methods for obtaining drugs that make up the group, methods of pharmaceutical analysis based on the physical and chemical properties of substances, and establishing a connection between chemical structure and pharmacological action.
All drugs are divided into inorganic and organic. Inorganic, in turn, are classified according to the position of the elements in the PS. And organic ones are divided into derivatives of the aliphatic, alicyclic, aromatic and heterocyclic series, each of which is divided into classes: hydrocarbons, halogen derivatives of hydrocarbons, alcohols, aldehydes, ketones, acids, ethers and esters, etc.
ALIPHATIC COMPOUNDS, LIKE DRUGS.
Preparations of aldehydes and their derivatives. Carbohydrates
Aldehydes
This group of compounds includes organic medicinal substances containing an aldehyde group or their functional derivatives.
General formula:
Pharmacological properties
The introduction of an aldehyde group into the structure of an organic compound gives it a narcotic and antiseptic effect. In this regard, the action of aldehydes is similar to the action of alcohols. But unlike the alcohol group, the aldehyde group increases the toxicity of the compound.
Factors influencing the structure on the pharmacological action :
elongation of the alkyl radical increases activity, but at the same time toxicity increases;
the introduction of unsaturated bonds and halogens has the same effect;
the formation of the hydrated form of aldehyde leads to a decrease in toxicity. But the ability to form a stable hydrate form is manifested only in chlorinated aldehydes. Thus, formaldehyde is a protoplasmic poison, used for disinfection, acetaldehyde and chloral are not used in medicine due to their high toxicity, and chloral hydrate is a drug used as a sleeping pill and sedative.
The strength of the narcotic (pharmacological) effect and toxicity increased from formaldehyde to acetaldehyde and chloral. The formation of the hydrate form (chloral hydrate) can dramatically reduce toxicity while maintaining the pharmacological effect.
According to physical condition aldehydes may be gaseous (low molecular weight), liquids and solids. Low molecular weight ones have a sharp unpleasant odor, high molecular weight ones have a pleasant floral odor.
Chemical properties
Chemically, these are highly reactive substances, which is due to the presence of a carbonyl group in their molecule.
The high reactivity of aldehydes is explained by:
a) the presence of a polarized double bond
b) carbonyl dipole moment
c) the presence of a partial positive charge on the carbonyl carbon atom
σ -
σ + H
The double bond between C and O, unlike the double bond between two carbons, is highly polarized, since oxygen has a much higher electronegativity than carbon, and the electron density of the π bond is shifted towards oxygen. Such high polarization determines the electrophilic properties of the carbon of the carbonyl group and its ability to react with nucleophilic compounds (to enter into nucleophilic addition reactions). The oxygen group has nucleophilic properties.
Characteristic reactions are oxidation and nucleophilic addition
I. Oxidation reactions.
Aldehydeseasily oxidize. Oxidation of aldehydes to acids occurs under the influence as strongand weak oxidizing agents .
Many metals - silver, mercury, bismuth, copper - are reduced from solutions of their salts, especially in the presence of alkali. This distinguishes aldehydes from other organic compounds capable of oxidation - alcohols, unsaturated compounds, the oxidation of which requires stronger oxidizing agents. Consequently, the oxidation reactions of aldehydes with complexly bound cations of mercury, copper, and silver in an alkaline medium can be used to prove the authenticity of aldehydes.
I. 1 .Reactionwith ammonia solution of silver nitrate (silver mirror reaction) FS is recommended to confirm the authenticity of substances with an aldehyde group. It is based on the oxidation of aldehyde to acid and the reduction of Ag + to Ag↓.
AgNO 3 + 2NH 4 OH → NO 3 +2H 2 O
NSSON+ 2NO 3 + H 2 O → HCOONH 4 + 2Ag↓+ 2NH 4 NO 3 + NH 3
Formaldehyde, oxidizing to the ammonium salt of formic acid, reduces metallic silver, which is precipitatedon the walls of the test tube in the form shiny coating "mirror" or gray sediment.

I. 2. Reactionwith Fehling's reagent (a complex compound of copper (II) with potassium-sodium salt of tartaric acid). Aldehydes reduce the copper(II) compound to copper(I) oxide, A brick-red precipitate forms. Prepare before use).
Felling's reagent 1 - CuSO 4 solution
Felling's reagent 2 – alkaline solution of potassium-sodium salt of tartaric acid
When mixing 1:1 Felling's reagents 1 and 2 a blue copper complex compound is formed (II) with potassium-sodium tartaric acid:

blue coloring
When the aldehyde is added and heated, the blue color of the reagent disappears, and an intermediate product is formed - a yellow precipitate of copper (I) hydroxide, which immediately decomposes into a red precipitate of copper (I) oxide and water.
2KNa+ R- COH+2NaOH+ 2KOH→ R- COONa+4KNaC 4 H 4 O 6 + 2 CuOH ↓ +H2O
2 CuOH ↓ →Cu 2 O ↓ +H2O
Yellow sediment brick red sediment

The textbooks have a different general reaction scheme
I. 3. Reactionwith Nessler's reagent (alkaline solution of potassium tetraiodomercurate (II). Formaldehyde reduces the mercury ion to metallic mercury - a dark gray precipitate.
R-COH + K 2 +3KOH → R-COOK + 4KI + Hg↓ + 2H 2 O




